top of page
Blog Tool
Search


Sensydia Announces American Medical Association Category III CPT® Code for Noninvasive AI Hemodynamic Assessment
Sensydia Announces American Medical Association Category III CPT® Code for Noninvasive AI Hemodynamic Assessment. Groundbreaking code will facilitate use, adoption, and pathway for reimbursement of noninvasive AI hemodynamic assessment, including Sensydia’s Cardiac Performance System (CPS™). The new CPT code, 1036T, was published December 30, 2025, and will be effective July 1, 2026.
Admin
Jan 52 min read


Sensydia Awarded Second Phase of $3M NIH Grant Award to Demonstrate Clinical Utility of Cardiac Performance System (CPS™)
The NIH has activated Phase II funding of Sensydia’s Fast-Track STTR grant to support real-world clinical utility studies of the Cardiac Performance System (CPS™). This non-dilutive funding marks a key milestone as the company continues to validate CPS for patients with heart failure and pulmonary hypertension.
Admin
Jun 9, 20252 min read


Sensydia Appoints Rusty Page as President and Chief Operating Officer to Support Growth and Commercialization of the Cardiac Performance System (CPS™)
Industry veteran brings over 20 years of medical device leadership to guide operations, quality, and infrastructure as Sensydia advances toward FDA submission and commercialization of the Cardiac Performance System (CPS™).
Admin
May 6, 20252 min read

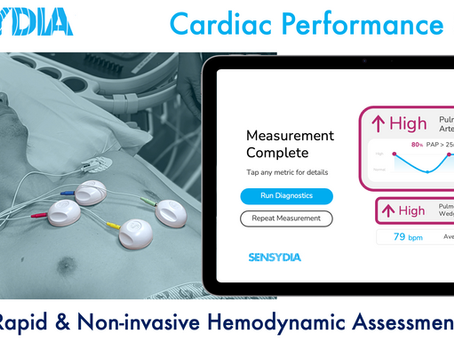
Sensydia Announces First Patient Enrolled in Pivotal Study of Non-Invasive Cardiac Performance System (CPS™)
Sensydia has enrolled the first patient in its pivotal clinical study evaluating the Cardiac Performance System (CPS™), a non-invasive solution for hemodynamic assessment. This milestone advances CPS™ toward FDA submission and supports Sensydia’s mission to transform the management of heart failure and other cardiac conditions through faster, safer diagnostics.
Admin
Apr 28, 20252 min read


Clinical data presented at ACC 2025 shows Sensydia CPS™ provides accurate assessment of mean pulmonary artery pressure non-invasively
Completion of this 50-subject study is a key milestone on the path to delivering the first device for non-invasive and accurate...
Admin
Mar 29, 20253 min read


Sensydia Completes Fifth Study for Heart-Sound AI
Completion of this 50-subject study is a key milestone on the path to delivering the first device for non-invasive and accurate...
Admin
Jan 16, 20243 min read


Sensydia secures $3M NIH grant to advance AI-based cardiac assessment platform
NIH STTR Fast-Track grant helps propel Sensydia's non-invasive CPS platform building on recent funding round Los Angeles, CA – June 5,...
Admin
Jun 5, 20232 min read


Sensydia raises $8M round to advance breakthrough CPS cardiac assessment platform
Sensydia continues to push the boundaries of AI in heart function assessment Los Angeles, CA – May 9, 2023 – Non-invasive cardiac...
Admin
May 9, 20233 min read


Sensydia Achieves Study Target for Heart-Sound AI
Completion of enrollment for this 225-subject study is a key milestone on the path to delivering the first device for non-invasive and...
Admin
Feb 6, 20233 min read


Sensydia Receives FDA Breakthrough Device Designation for CPS Non-Invasive Cardiac Monitoring Device
External device uses heart sound and AI to deliver rapid cardiac function assessments via tablet to expand hemodynamic evaluation beyond...
Admin
Jan 19, 20222 min read


Sensydia Appoints Anthony Arnold as President and Chief Executive Officer
Los Angeles – October 1, 2019 – Sensydia Corporation, an innovator in rapid, non-invasive measurement of critical cardiac function,...
Admin
Oct 1, 20192 min read


FDA Clears Sensydia’s Cardiac Performance System
The Sensydia Cardiac Performance System (CPS) is the first patient-worn system to provide Ejection Fraction information to clinicians for...
Admin
Jun 18, 20182 min read


Sensydia Completes Series B Financing
Sensydia Corporation announces that it has complete a Series B financing. The financing was led by Colle Capital and included all Series...
Admin
Jul 1, 20171 min read


Sensydia Completes Series A Financing
Sensydia Corporation has completed its first round of financing which will be used to develop its patent pending technology to detect...
Admin
Apr 4, 20161 min read
bottom of page